Significant updates were presented in ASCO and EHA for the treatment of patients newly diagnosed disease.
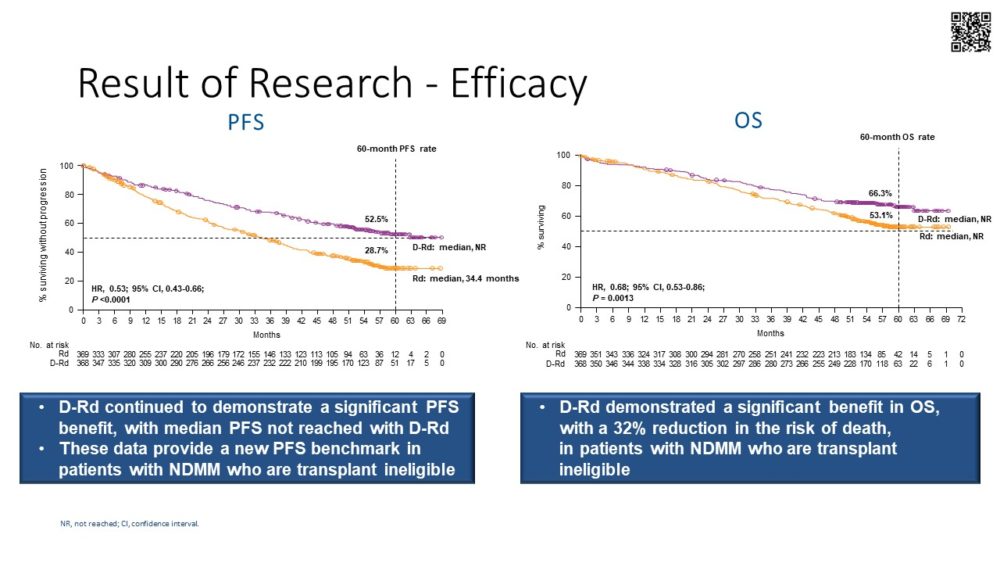
In the late breaking abstract session, Prof. T. Facon presented the results on overall survival from the MAIA study comparing Dara-Rd vs Rd in newly diagnosed transplant ineligible patients (Abstract LB1901). The 60-month PFS rate was 52.5% for Dara-Rd vs 28.7% for Rd, and the 60-month OS was 66.3% vs 53.1% (HR: 0.68) and was observed across all predefined subgroups (except those with hepatic dysfunction and was less in those with high risk cytogenetics). Importantly, 15% of patients in the Dara-Rd arm and 46% in the Rd arm received daratumumab in any subsequent line of therapy. There were no new safety concerns with the additional follow up. This study provided the longest PFS and OS rates reported so far for patients with newly diagnosed myeloma who are not transplant eligible.
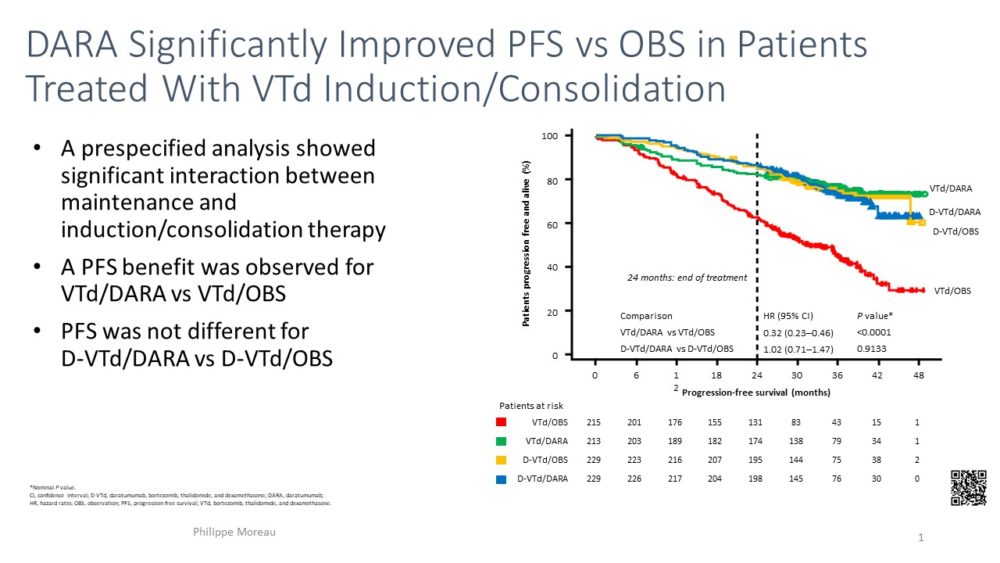
Prof P. Moreau presented the updated CASSIOPEIA data , in both ASCO (abstract 8004) and EHA (Abstract S180) meetings, that compared VTD plus daratumumab vs VTD induction (1st randomization) and daratumumab maintenance vs observation (2nd randomization) post ASCT. The presentation focused on results from 2nd randomization. Addition of daratumumab improved PFS, with a HR of 0.53 (95%CI 0.42-0.68), in all prespecified subgroups. The two randomizations of the study (one at induction and a second at maintenance) allowed to evaluate the benefit of daratumumab at different settings. Importantly the benefit of added daratumumab was also observed even when given only in induction and not as maintenance or when given only as maintenance.